Proton beam on / Particle physicists pursue protons for medical imaging
A new kind of detector technology that could lead to discoveries in particle physics may also lead to better 3D images of the human body and help cancer patients.
By Elizabeth Clements
Continuing a tradition of collaboration between particle physics and medicine, researchers from Northern Illinois University are teaming with physicists from Fermilab and Argonne national laboratories to build a new type of CT scan detector that would use protons instead of the traditional X-rays.
Physicians use CT, or computed tomography, scans to create a 3D image of a body to diagnose diseases, such as cancer, and plan a course of treatment. Scientists believe that a proton CT scan could offer more accurate imaging and therefore more effective treatment.
"We will get a more accurate anatomical image of the density map within the body," says George Coutrakon, a medical physicist at NIU. "X-rays do this fairly well, but errors associated with the X-ray CT scan can be reduced by using protons."
Applications in cancer treatment
One area where proton CT scans could be very useful is just before cancer treatmentespecially treatment that uses protons.
National laboratories are not strangers to proton therapy. Fermilab's first director, Robert Wilson, wrote the seminal paper in 1946 showing that protons could be an effective method for treating cancer.
While at lower doses both X-rays and protons can be used to image the body, at high doses, Wilson noted, they both can be used to destroy a tumor. But they differ in the way they work.
When an X-ray moves through the human body, it leaves energy deposits along its path. As a result, the X-rays affect not only the tumor but also any organs or tissue they travel through along their way, leading to potential damage to healthy body parts and increased side effects. A proton, however, releases the majority of its energy at the end of its path, making it a perfect precision tool for physicians. By adjusting the speed of the proton, a physician can pinpoint where it deposits its energy to kill the tumorand only the tumor. As a result, for certain kinds of cancers, such as brain tumors and pediatric tumors, proton therapy can be a better option than traditional X-ray radiation.
The first proton therapy patient was treated in 1954 at Lawrence Berkeley National Laboratory's 184-Inch Cyclotron accelerator. In the 1960s, scientists at the Harvard Cyclotron, another accelerator originally designed for physics experiments, began using proton beams to precisely target tumors with minimal impact on the surrounding organs.
Since then, protons have been used as the optimal way to treat certain kinds of cancer; nine proton therapy centers currently offer the treatment in the United States, and more centers are under construction and planned.
3D-imaging with protons
Proton therapy treatment is only as good as the imaging guiding it. Today, proton therapy patients receive CT scans with X-rays a day or two prior to treatment to map out the current size of the tumor.
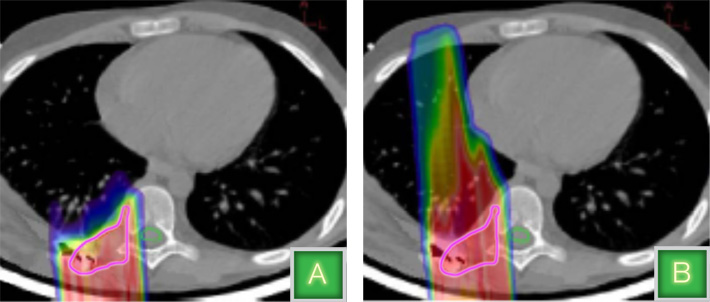
In a traditional CT scanning machine, a patient lies down on a platform that slides into a donut-like ring. A tube mounted on one side of the ring generates X-rays, and a detector on the other side records the data. The ring rotates around the patient, shooting X-rays from enough angles to create a 3D image. Then, the scans are used to determine the radiation dose needed to treat the tumor. For a proton therapy patient, a CT scan that uses X-rays allows, on average, a three percent error in the depth at which the protons stop.
"To accommodate that error, physicians typically extend the treatment volume to make sure that the tumor is adequately covered; sometimes that means you are treating healthy tissue and increasing risk of complications," Coutrakon says. "We believe that with a proton CT we can reduce that margin to one percent, using at least three to five times less dose than an X-ray CT."
A CT scan that uses protons instead of X-rays would create an image that would allow physicians to precisely target the affected area and treat only the tumor.
Although scientists first envisioned harnessing the proton's precision for medical imaging in the 1960s and 1970s, they've been unable to do so until now.
"It's been a long time in coming because of the substantial R&D that needed to be accomplished," Coutrakon says.
Coutrakon explains that a proton CT scan could be done on any part of the body, but it would have its biggest advantages with tumors near critical structures in the body, such as the spinal cord or optic nerves.
"In some cases, physicians will be able to increase the dose of radiation to the target while reducing toxic side effects if they have better imaging," he says.
Collaboration with national laboratories
Four years ago, NIU joined a collaboration between the University of California, Santa Cruz and the proton treatment center at Loma Linda University Medical Center, a pioneer in the field of proton therapy, to build and test a first-generation proton system. The prototype system allowed the team to demonstrate the advantages of a proton CT scan.
Now the NIU team is working with Fermilab and Argonne on a second-generation model to adapt the technology for clinical use. This new collaboration, which also has international participants from the University of Delhi in India, is aimed at bringing the technology much closer to clinical utility, which requires large-area detectors, shorter scan times, and faster image reconstruction.
Scientists at NIU and the two Department of Energy national laboratories are building a next-generation detector and imaging system that will be able to scan and reconstruct an area comparable to the size of a human head in 10 minutes, making it clinically useful. Fast data acquisition and image reconstruction are the two main focuses for the collaboration.
Better particle detectors: silicon photomultipliers
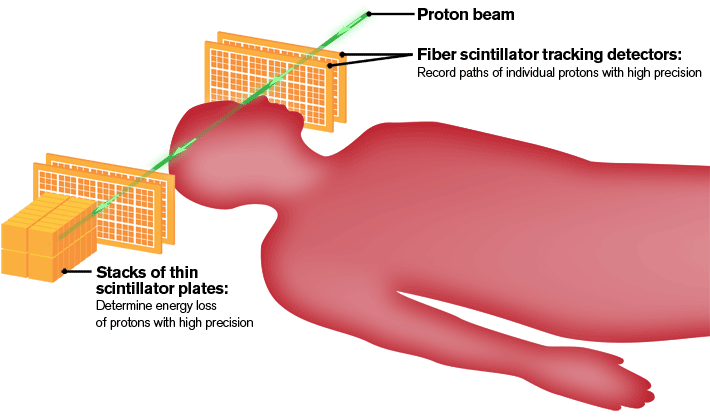
In order to precisely reconstruct images of the tumor and neighboring anatomy, the detector will use a fiber tracker, the ultimate GPS device in particle physics, to pinpoint the exact location where each individual proton enters and exits the target area. While a GPS system uses satellites to determine locations, the tracker will use scintillating fibers to detect the path of protons. Photomultipliers will see light produced by the scintillating fibers and send the information to computers for analysis.
Photomultipliers made out of silicon, called SIPMs, offer many advantages for particle detectors. That's why, in addition to medical imaging, physicists are developing them for future high-energy physics experiments. They are already used extensively on the T2K experiment in Japan and are being developed for upgrades to the CMS experiment at CERN in Switzerland.
"This is the first time we are trying to build a tracker with SIPMs to read out the data," says Peter Wilson, a physicist leading the effort to build the proton tomography detector at Fermilab. "It's a step toward what we will do in the future for high-energy physics experiments, and there is a lot we can learn from assembling this first detector."
In addition to preparing the scintillating fibers and other components for the tracker, Fermilab will develop the electronics to read out the data.

Instant results thanks to advanced computing
With a goal of recording two million particle events a second, the team is also developing advanced software to reconstruct the 3D images in a reasonable timeframe.
"The current image reconstruction time is 10 to 20 hours," Coutrakon says. "The goal is to redevelop the code and reconstruct a 3D image in 10 to 20 minutes."
After re-engineering the software developed by the first collaboration to run on an advanced computing cluster, scientists from NIU and the Mathematics and Computer Science Division at Argonne National Laboratory have already reduced the calculation time to 33 minutes. They're quickly approaching their 10-minute goal.
Besides giving images for more accurate proton treatment planning, proton CT would also be useful for immediate verification of the plan before treatment.
"With today's technology a patient would need to come in a day or two prior to treatment for a proton CT scan because it takes an entire day to create the image," says Nick Karonis, a computer scientist at NIU and Argonne. "It would be valuable to have an image just minutes before the treatment to confirm that the treatment plan still makes sense. We want to get the time down to make that clinically possible."
Preparing for clinical trials
The team plans to complete the next-generation detector by the end of 2012. From there, scientists will try out the detector in a test beam at Loma Linda for about a year. For this stage of testing, the scientists will use plastic versions of body parts that have physical properties that mimic those in the human body.
Once the team completes the beam tests, they will install the detector on a gantry, the ring that rotates around the patient to capture the 3D image. Including the time it will take to gain Food and Drug Administration approval, the project should be complete in about five years.






